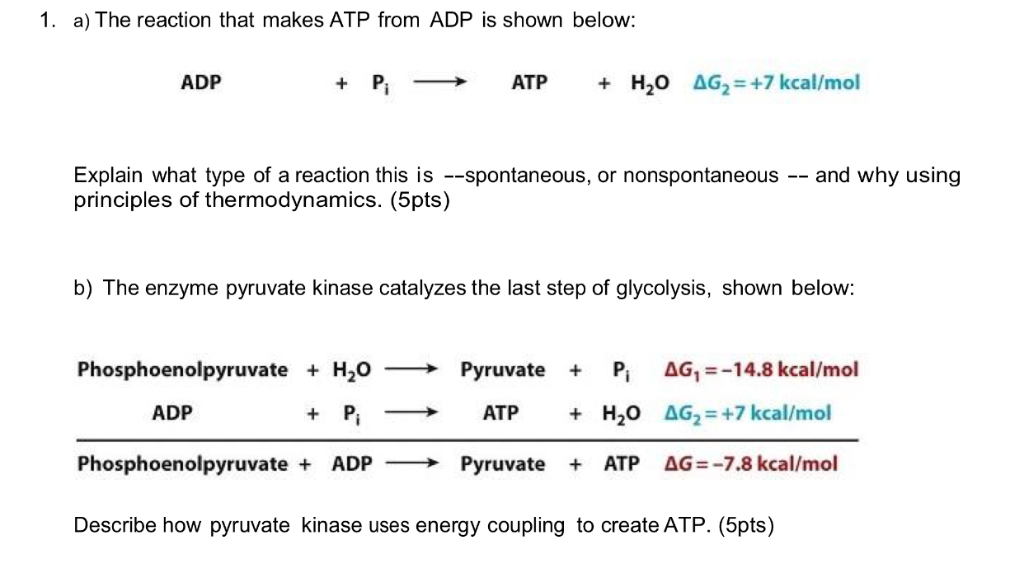
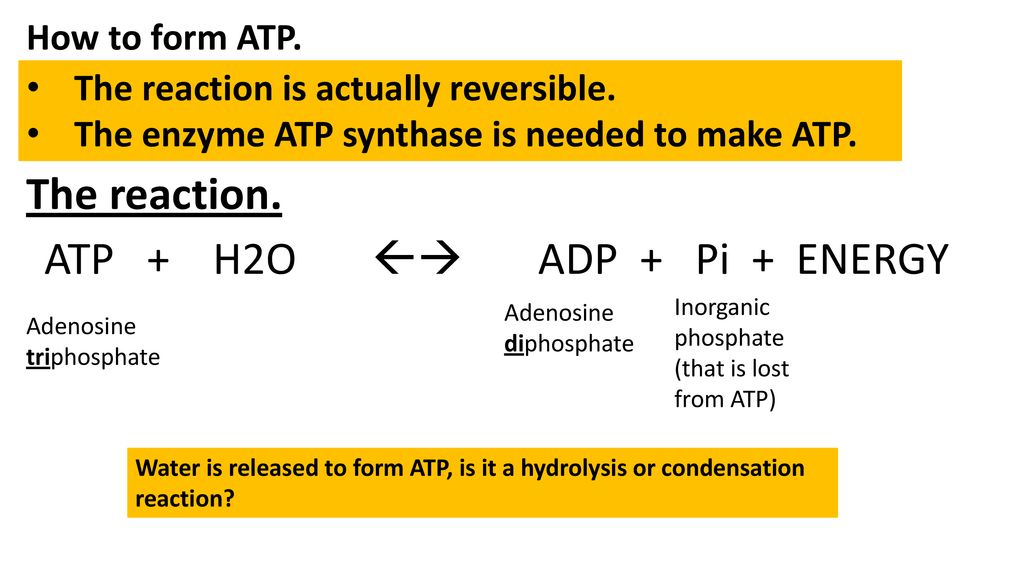
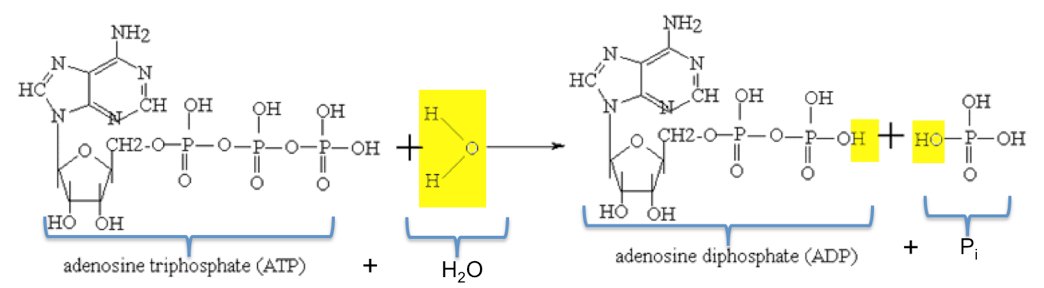
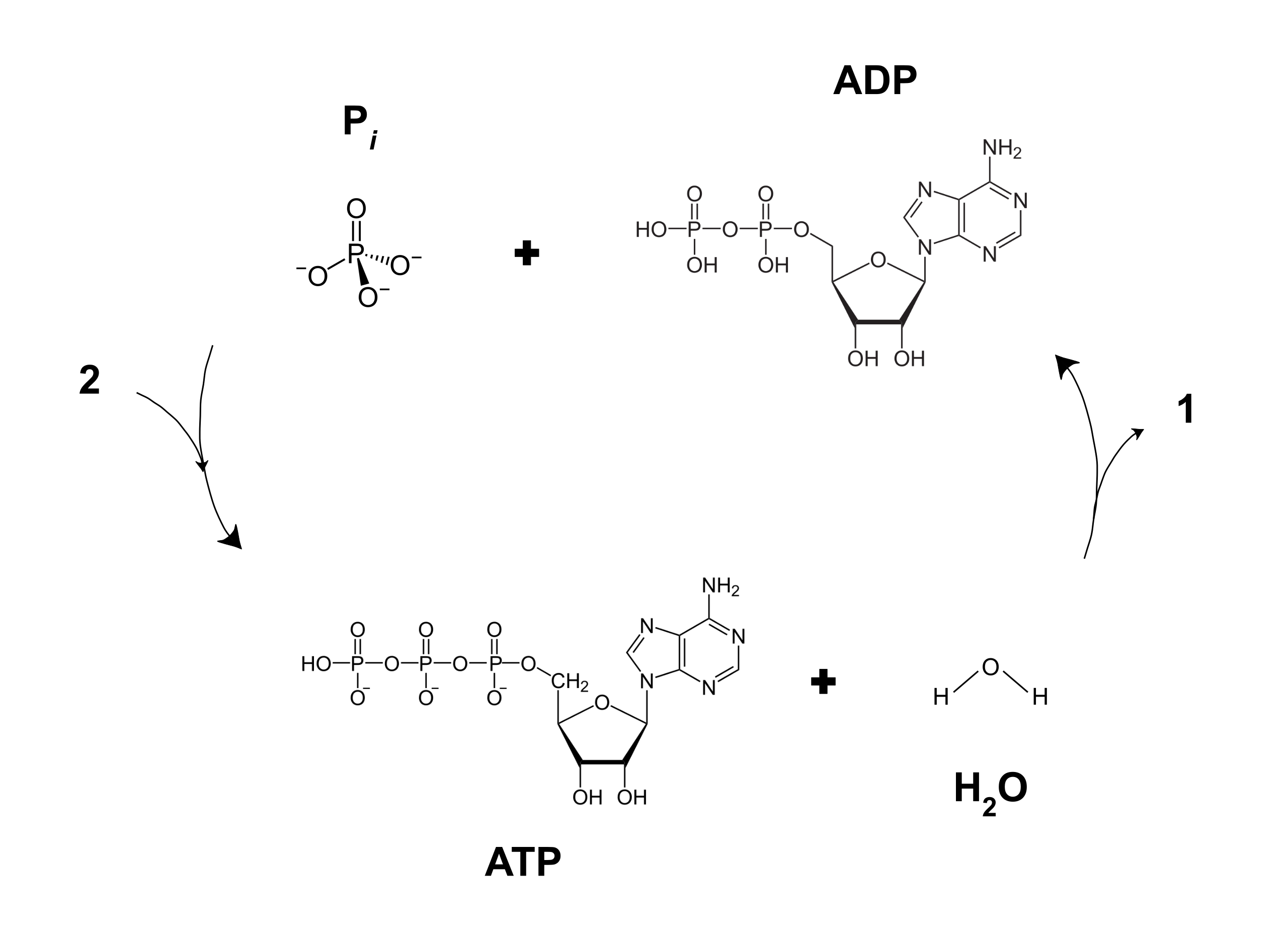
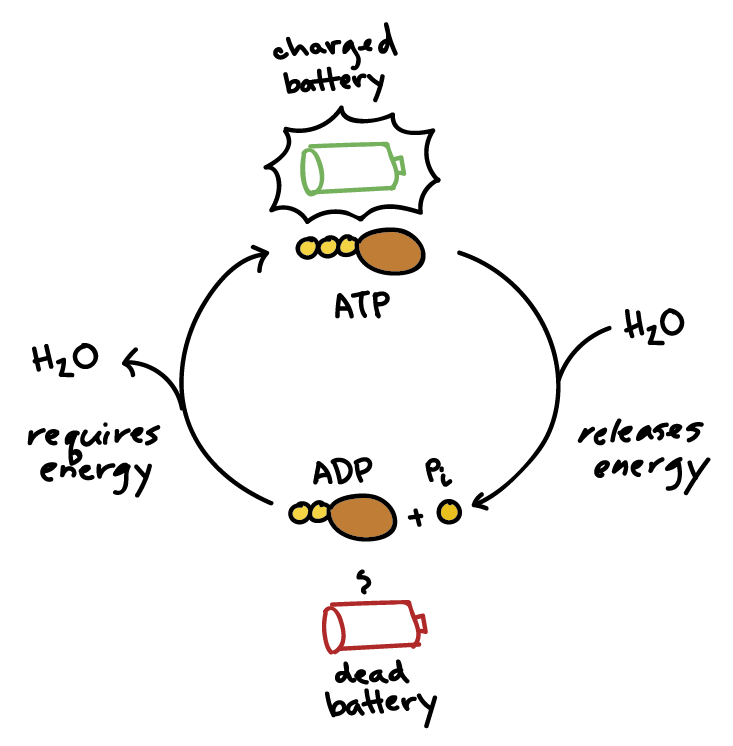
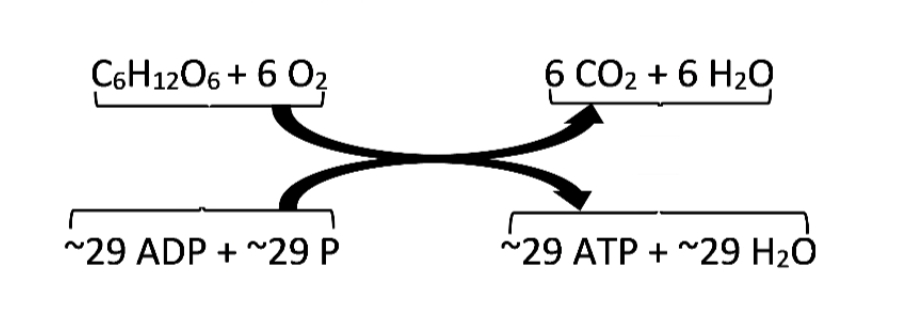
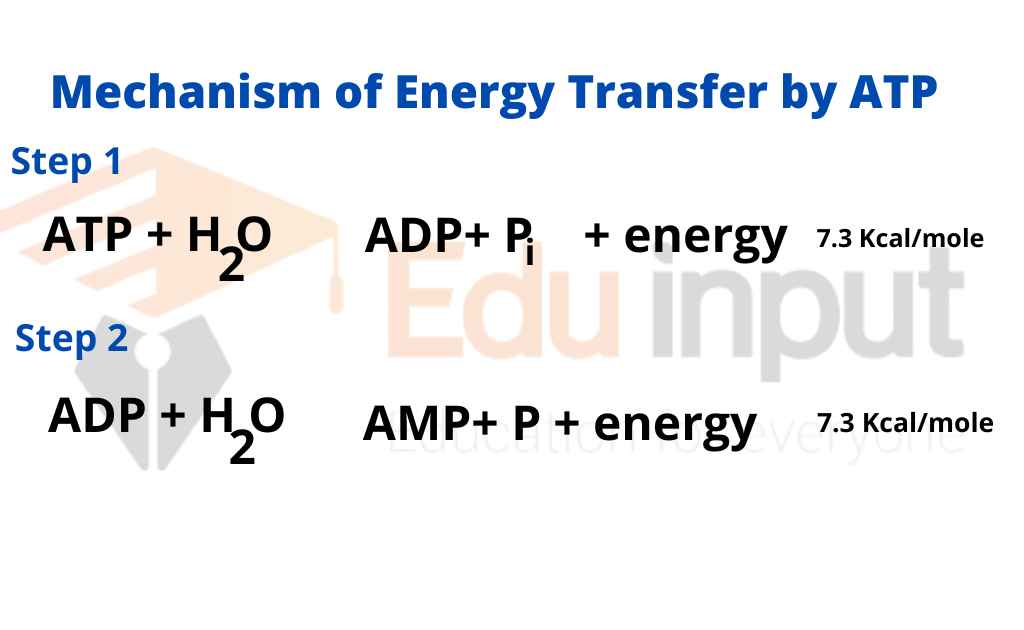
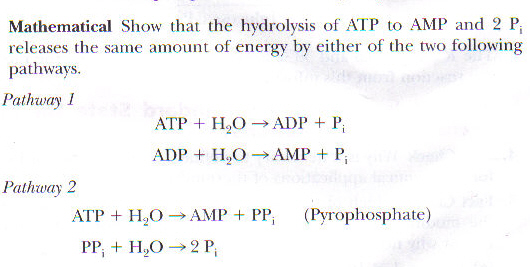
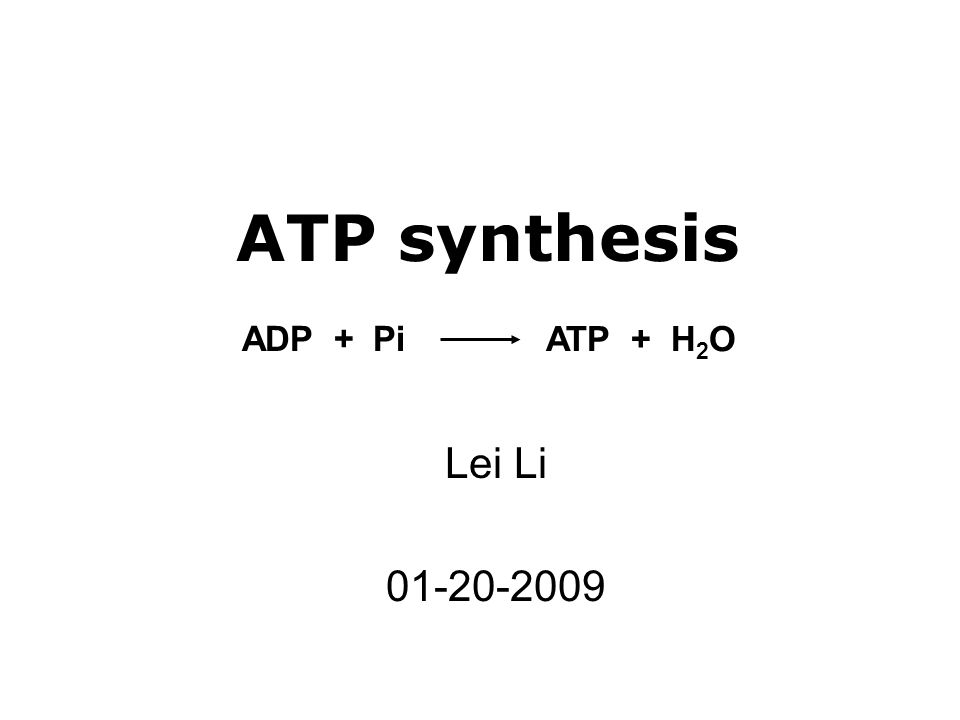
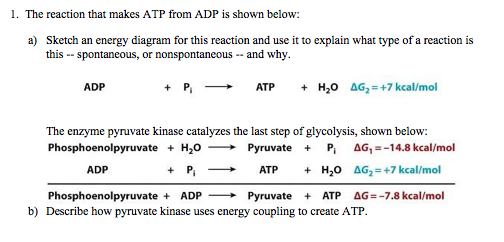
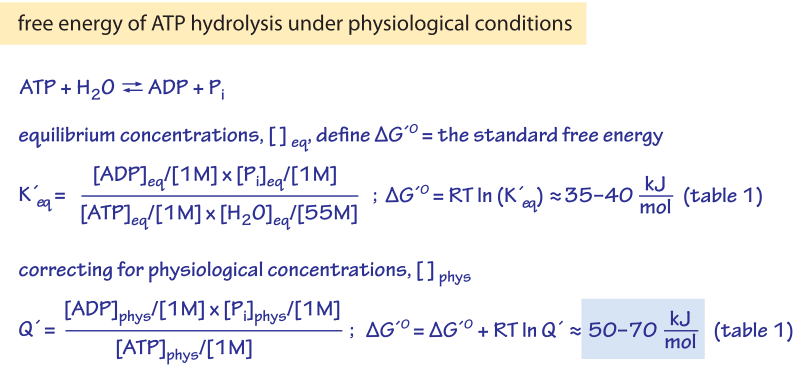
3. Give one reason why the reaction, ADP + P > ATP + H20, requires energy input. (Hint: Notice the - Brainly.com

Hydrolysis of ATP to ADP and inorganic phosphate (P i ) by reaction... | Download Scientific Diagram
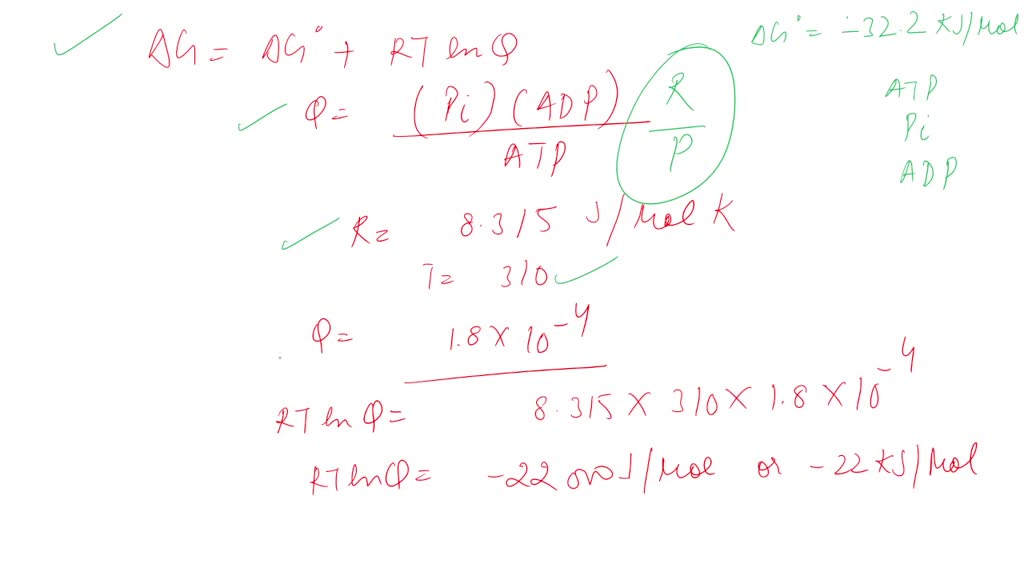
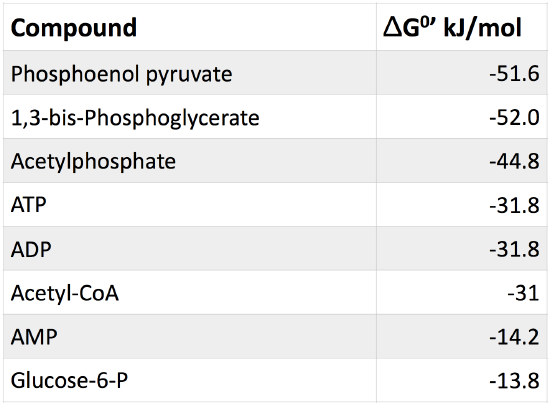
SOLVED: The standard free energy of hydrolysis (∆G°´) of ATP is -32.2 kJ/mol. ATP + H2O → ADP + Pi The actual free energy of ATP hydrolysis, represented by ∆G, depends on

A water molecule is added to an atp molecule to break atp down into adp and a phosphate group. write the - Brainly.com

SOLVED: In the reaction catalyzed by hexokinase, the two half reactions and their ΔG° values are as follows: ATP+H2O↔ADP+Pi ΔG°= -31 KJ. mol-1 Pi+glucose↔glucose-6-P+ H2O ΔG°= +14 KJ. mol-1 The ΔG° conversion