bluebird bio Announces FDA Approval of ZYNTEGLO®, the First Gene Therapy for People with Beta-Thalassemia Who Require Regular Red Blood Cell Transfusions - bluebird bio, Inc.
Bluebird bio's gene therapy Zynteglo could have high price tag defense in beta thalassemia - Pharmaceutical Technology

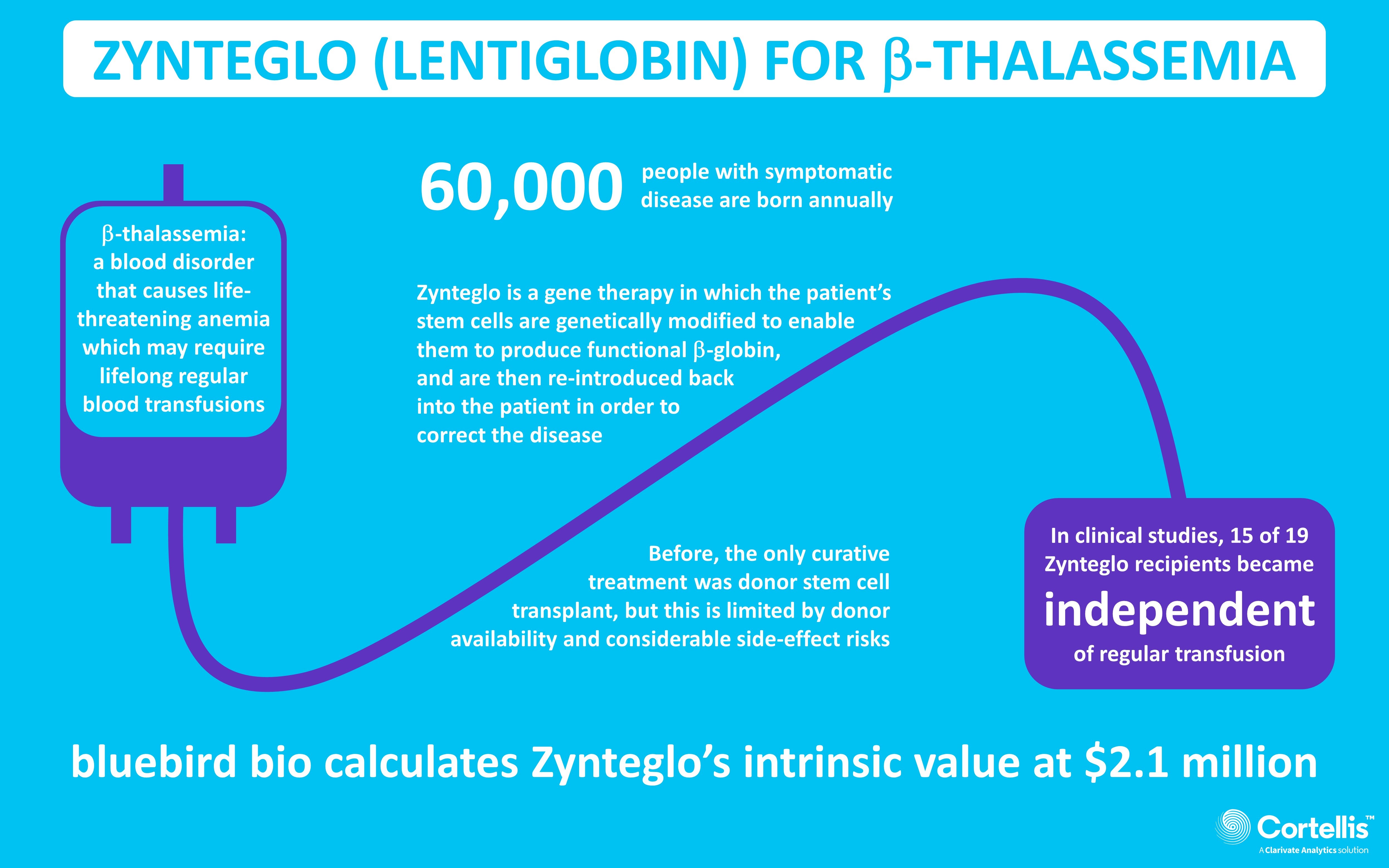
ICER Draft Evidence Report on Bluebird Gene Therapy Zynteglo Finds It Provides Value at $2.1 Million - Global Genes

bluebird bio Announces FDA Approval of ZYNTEGLO®, the First Gene Therapy for People with Beta-Thalassemia Who Require Regular Red Blood Cell Transfusions | Business Wire

bluebird bio Announces EU Conditional Marketing Authorization for ZYNTEGLO™ (autologous CD34+ cells encoding βA-T87Q-globin gene) Gene Therapy for Patients 12 Years and Older with Transfusion-Dependent β-Thalassemia Who Do Not Have β0/β0 Genotype
Bluebird bio sets €1.6M price tag for rare blood disease gene therapy | S&P Global Market Intelligence

ICER's Favorable Assessment Of Bluebird Bio's Gene Therapy Zynteglo May Have Important Pricing And Reimbursement Implications

FDA approves bluebird bio's Zynteglo gene therapy for beta thalassemia; shares up 7% | Seeking Alpha