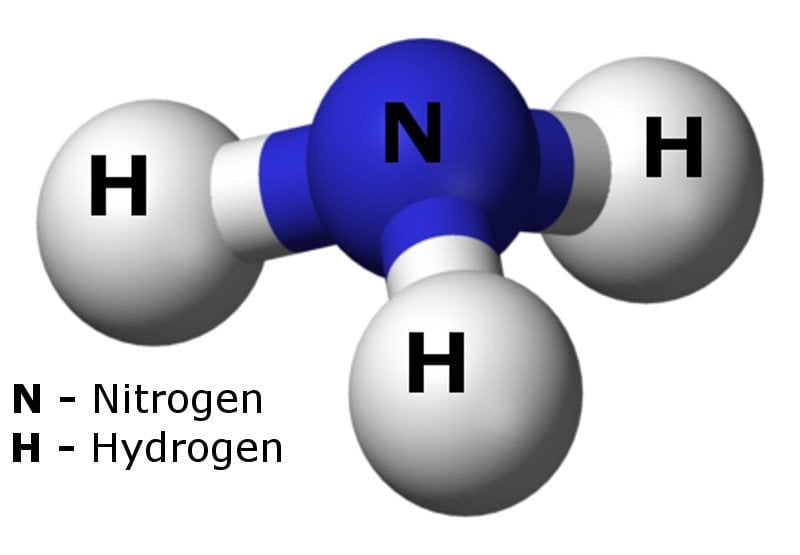
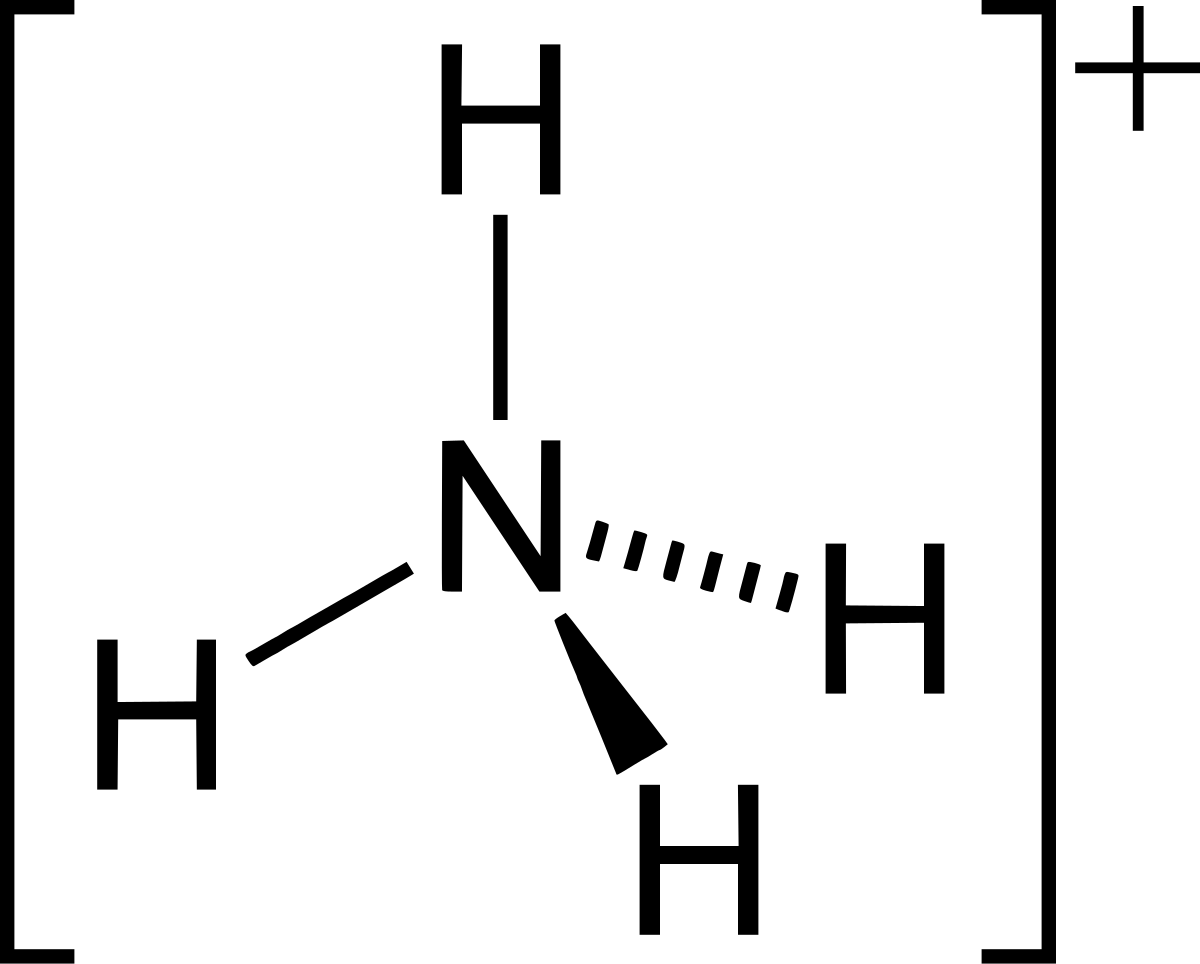
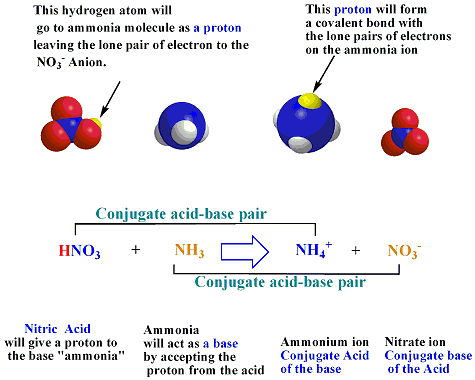
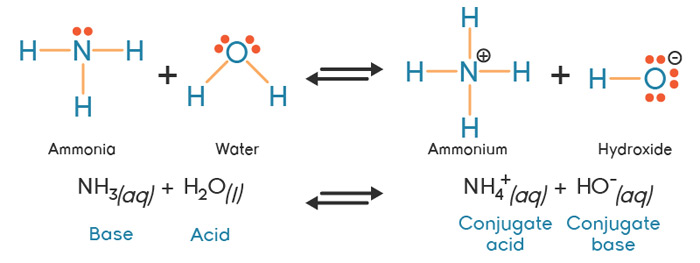
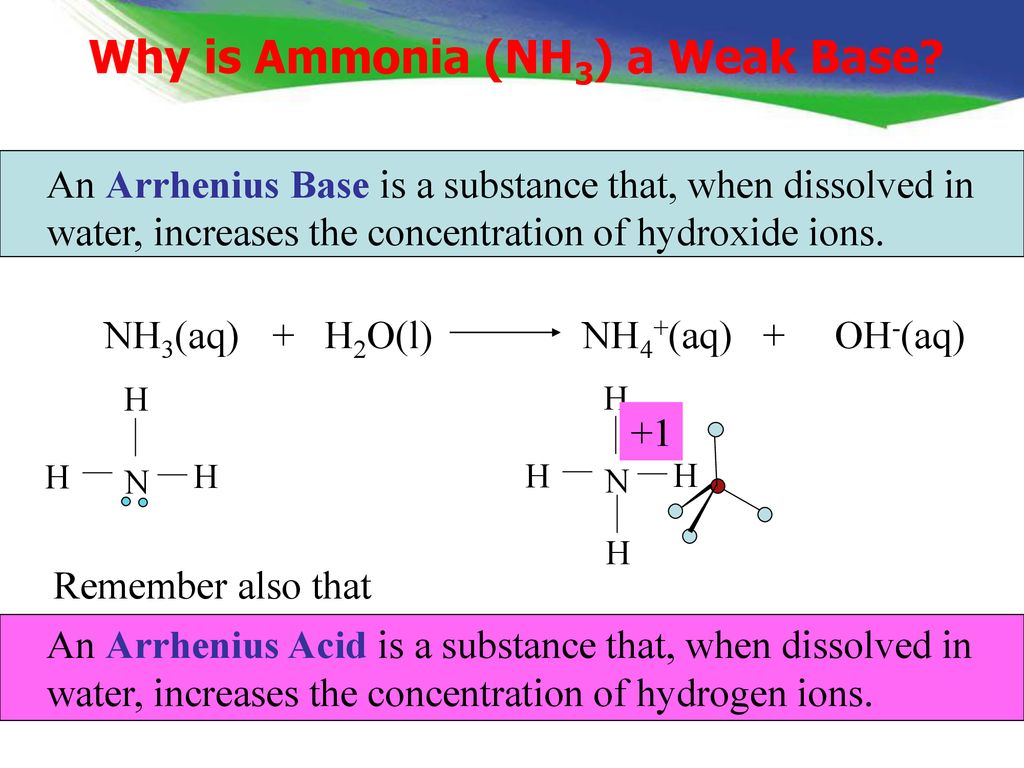
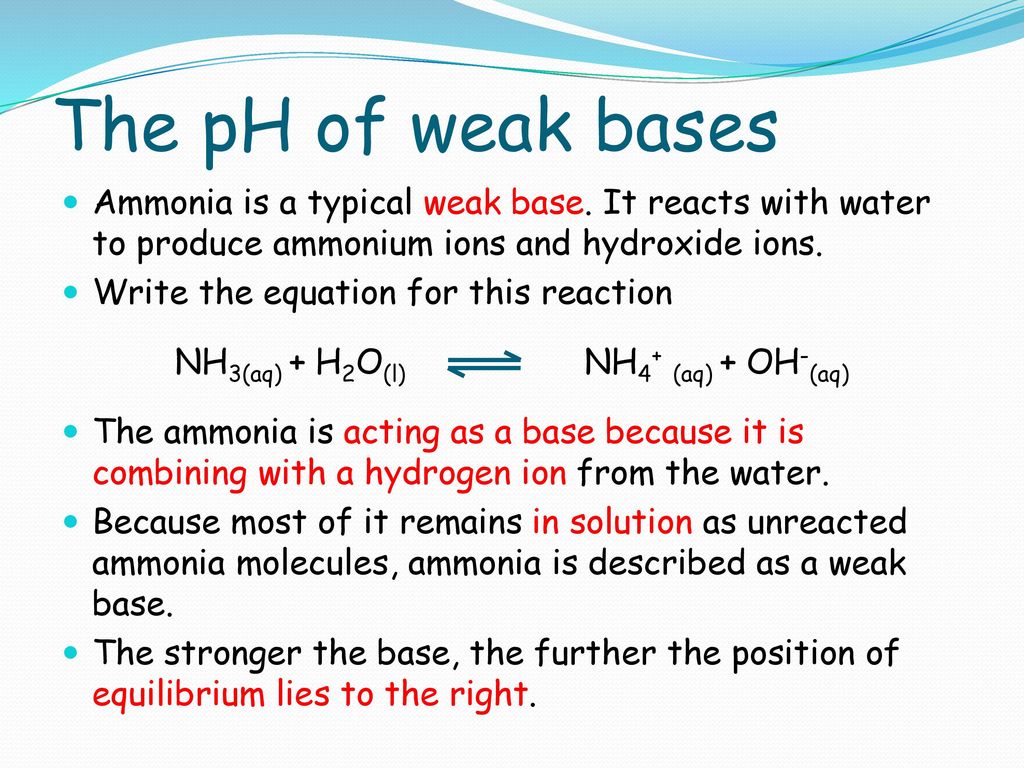
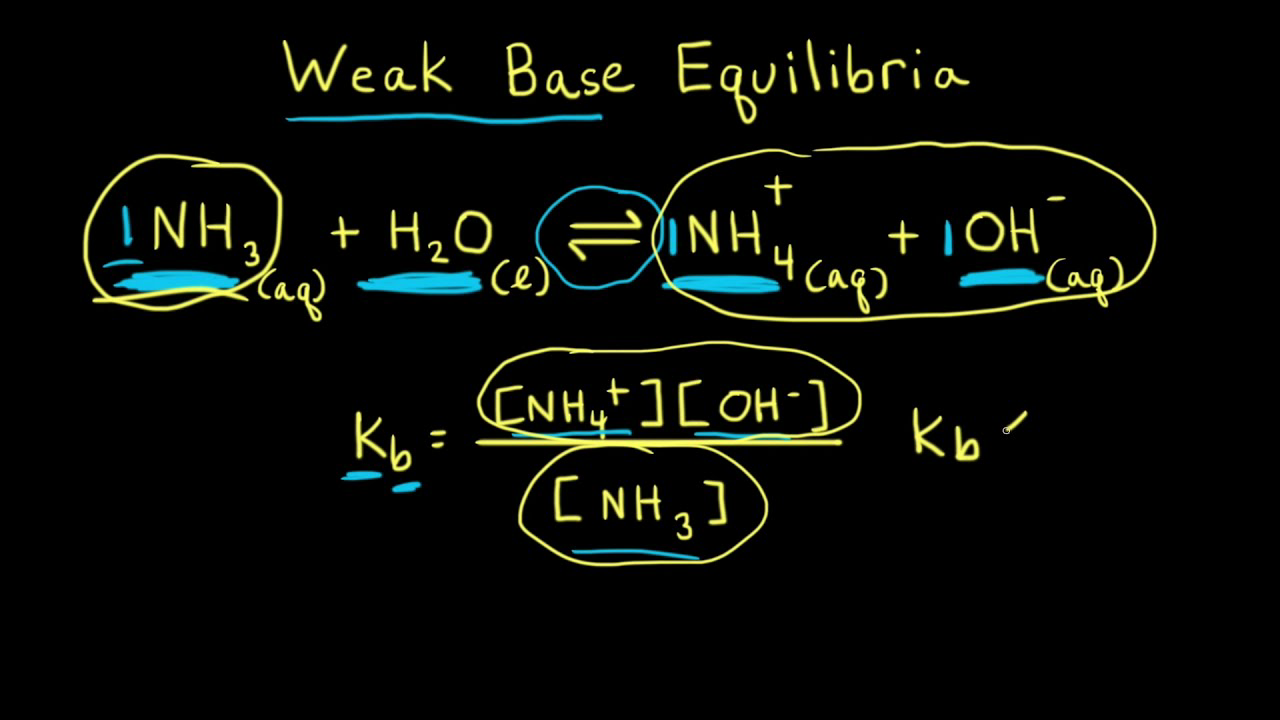Spontaneity of the acid–base reaction between acetic acid and ammonia... | Download Scientific Diagram

Question Video: Identifying the Lewis Acid in the Reaction of Ammonia with Boron Trifluoride | Nagwa
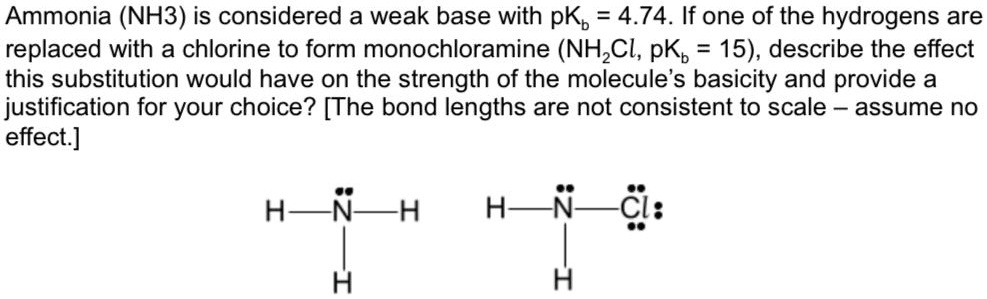
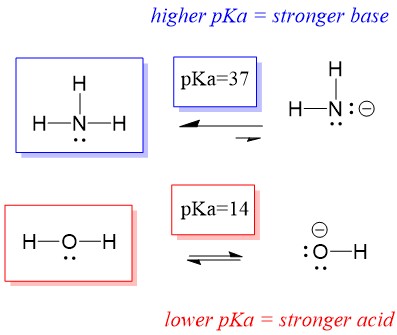
SOLVED: Ammonia (NH3) is considered a weak base with pKb = 4.74. If one of the hydrogens are replaced with a chlorine to form monochloramine (NHZCl, pKb 15) , describe the effect